Abstract
Background: Allogeneic stem cell transplantation (allo-SCT) has become an increasingly important consolidation treatment option for patients with acute myeloid leukemia (AML) and as upfront therapy for patients with high-risk myelodysplastic syndrome (MDS). Although the median age at diagnosis for both diseases is above 65 years, studies evaluating allo-SCT as treatment option for patients aged 65 years or older are limited. Further, as the population ages, the number of patients above 65 years considered for allo-SCT will continue to rise. Thus, the aim of our current investigation was to analyze outcomes based on age in AML/MDS patients <65 years old and ≥65 years old who received allo-SCT at the Ohio State University.
Methods: A retrospective analysis was performed for all AML/MDS patients who received allo-SCT between January 1984 and December 2018 at our institution. Primary endpoints included progression free survival (PFS) and overall survival (OS). PFS was counted from the day of transplantation to relapse or death. OS was defined as survival from the day of allo-SCT until death from any cause, with censoring of patients known to be alive at the time of last follow-up. PFS and OS were calculated using Kaplan Meier Curves. Secondary endpoints included cumulative incidences of grade II-IV and III-IV acute GVHD (aGVHD), chronic GVHD (cGVHD), relapse, and non-relapse mortality (NRM). Cumulative incidence rates of aGVHD, cGVHD, relapse, NRM were estimated and compared using Gray's test accounting for competing risks.
Results: The cohort consisted of 900 AML/MDS patients, with 150 patients ≥65 years and 750 patients <65 years. The median age at transplant for the <65 years group was 49 years (range: 18-64 years) and 68 years (range: 65-76 years) for the ≥65 years group. Gender, race, Karnofsky score, and comorbidity index were similar between the two groups. A higher proportion of patients received myeloablative (MA) conditioning (65.1%) in the <65 years of age compared to 20% in the ≥65 years of age (p<0.01). A higher proportion of older patients had matched unrelated donors (57.3%), and reduced intensity conditioning (RIC) regimens (80%).
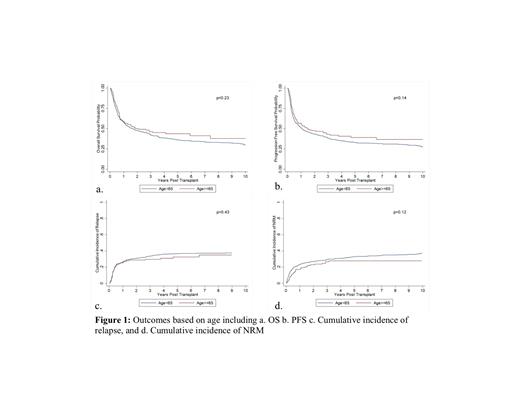
The median time from diagnosis to transplantation was 176 days (range: 55-4920) for age <65 years and 168 days (range: 34-6079 days) for age ≥65 years. Median follow-up from allo-SCT was 5.9 years (range 0.8-35.9 years) and 3.4 years (range: 1.0-9.6 years) from transplantation among survivors. Neutrophil and platelet engraftment were similar among the groups (p=0.35; 0.11). 3 year OS of 42.3% (95% CI: 38.7-45.8%) and PFS of 38.3% (95% CI: 34.8%-41.9%) were observed for age <65 years. The corresponding OS and PFS for age ≥65 years was 46.3% (95% CI: 37.9%-54.3%) and 43.0% (95% CI: 34.7%-51.0%), respectively (Figure 1a & 1b). Cumulative incidences of relapse at 1 year in <65 and ≥65 years were 26.4% and 25.3%, respectively (p=0.43). The cumulative incidence of NRM at 1 year in <65 and ≥65 years was 23.2% and 17.3%, respectively (p=0.12; Figures 1c and d). The incidences of acute and chronic GVHD were similar in the two age groups. The cumulative incidence of aGVHD at day 100 in <65 and ≥65 years was 40.3% (95% CI: 36.4%-44.2%) and 43.0% (95% CI: 34.9%-50.7%), respectively. The cumulative incidence of cGVHD at day 365 in <65 and ≥65 years was 40.8% (95% CI: 36.9%-44.6%) and 41.6% (95% CI: 33.6%-49.4%), respectively.
Conclusion: Overall, our study suggests similar outcomes for elderly patients undergoing allo-HCT as compared to their counterparts, which is in line with prior studies. This likely is due to advancements in the transplant field, including the development of RIC and alternative donors, which have allowed greater access to transplant for older adults. Utilization of allo-HCT is feasible and should be considered for AML/MDS patients ≥65 years. Further research is underway to evaluate the important determinants of health status in older patients undergoing allo-HCT and to ultimately help predict NRM (BMT CTN 1704).
Bumma: Amgen, Sanofi: Speakers Bureau; Janssen, Oncopeptides, Sanofi: Consultancy. Vasu: Seattle Genetics: Other: travel support; Boehringer Ingelheim: Other: Travel support; Kiadis, Inc.: Research Funding; Omeros, Inc.: Membership on an entity's Board of Directors or advisory committees. Jaglowski: Takeda: Consultancy; Juno: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy; Novartis: Consultancy, Research Funding. Mims: Syndax Pharmaceuticals: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Abbvie: Consultancy; Genentech: Consultancy; Kura Oncology: Consultancy; Leukemia and Lymphoma Society: Consultancy; Glycomemetics: Research Funding; Aptevo: Research Funding; Xencor: Research Funding; Daiichi-Saynko: Consultancy. Brammer: Celgene: Research Funding; Seattle Genetics: Speakers Bureau; Kymera Therapeutics: Consultancy. Saad: Incyte Pharmaceuticals: Consultancy; careDx: Consultancy; Amgen: Research Funding; Kadmon: Research Funding; OrcaBio: Research Funding; Magenta Therapeutics: Consultancy. de Lima: Miltenyi Biotec: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.